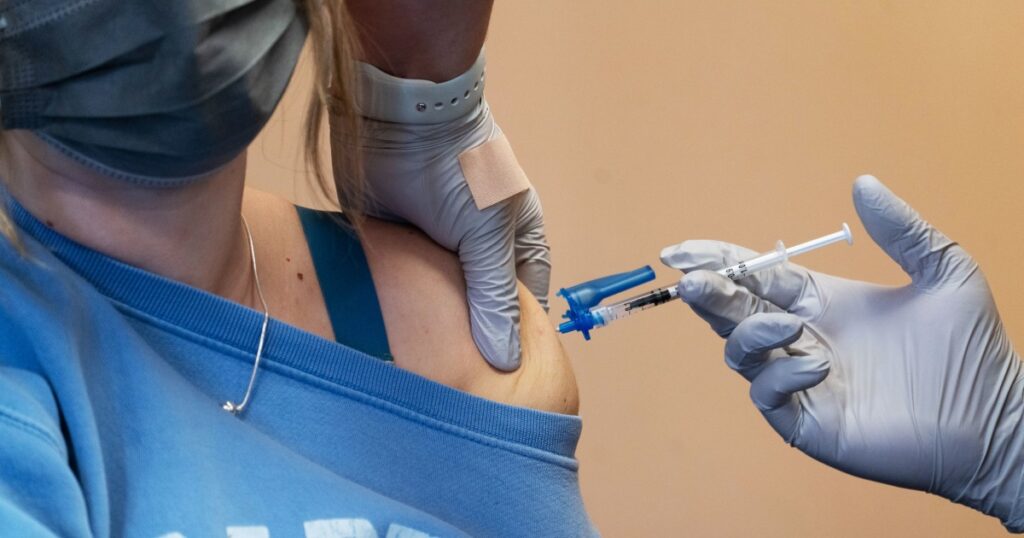
As respiratory virus season approaches in the United States, health officials are rolling out a fresh batch of updated COVID-19 boosters nationwide.
The new vaccines, which have been reformulated to target new variants, are recommended for everyone ages 6 months and older. Americans can expect the updated shots to become available as early as Friday in some parts of the country.
Earlier this week, the U.S. Food and Drug Administration authorized the updated mRNA vaccines for 2023-2024 from drugmakers Moderna and Pfizer-BioNTech.
Following the FDA’s green light, the U.S. Centers for Disease Control and Prevention’s advisory committee met on Tuesday, Sept. 12, to vote on recommendations for who should get the new booster and when. Advisers overwhelmingly recommended that everyone ages 6 months and older get the updated vaccines this fall to protect against potentially serious outcomes of COVID-19.
The CDC’s director Dr. Mandy Cohen signed off on the new recommendations Tuesday evening, kicking off a new nationwide COVID-19 vaccination campaign, NBC News reported.
“We have more tools than ever to prevent the worst outcomes from COVID-19,” Cohen said in a statement.
The broad vaccination recommendations come as the U.S. faces an uptick in infections and hospitalizations around the country. During the week ending on Sept. 2, 18,871 people were hospitalized with COVID, an 8.7% increase from the previous week, per the CDC.
What is the newest COVID-19 booster?
The newest COVID-19 booster is a vaccine that targets the omicron XBB.1.5 variant of the coronavirus.
Although many are referring to the updated vaccine as a booster, the shot looks different from its predecessors. It may be first of what will become an “annual COVID-19 shot” that gets revamped every year, similar to the seasonal influenza vaccine, Dr. Mark Mulligan, director of NYU Langone Vaccine Center, tells TODAY.com.
Unlike the last round of boosters, the updated mRNA vaccines are monovalent, which means they target a single variant. The previous vaccine rolled out last fall was bivalent, meaning it included two strains — the original SARS-CoV-2 virus and the omicron BA.4 and BA.5 subvariants, says Mulligan. The bivalent vaccine is no longer authorized by the FDA, the CDC said.
The XBB.1.5 subvariant targeted in the new vaccines was the dominant COVID strain in the U.S. for most of 2023. In recent months, it has been overtaken by new strains including the EG.5 or “Eris” subvariant, F.L.1.5.1 (also dubbed “Fornax”), and XBB.1.16 or “Arcturus” — which are all descendants of the omicron XBB lineage and close relatives of XBB.1.5, TODAY.com reported previously.
The updated vaccines are expected to provide good protection against the variants currently circulating, the FDA said.
Last month, a highly mutated new variant called BA.2.86 or “Pirola” gained global attention after health experts predicted it may be better able to bypass immunity from vaccination and prior infection. However, new data suggests that BA.2.86 may not be as immune-evasive as initially thought, and that the updated COVID shots will likely still be effective against the mutated strain.
Last week, Moderna said its updated COVID-19 vaccine prompts a strong immune response against BA.2.86 and generates neutralizing antibodies against other variants, including EG.5, and FL.1.5.1. Pfizer also announced that its reformulated vaccine produces an antibody response against the various omicron sublineages in circulation.
“The new vaccine is targeting subtypes of the omicron variant that not all of us have been exposed to yet … so it’s not quite a booster. This is trying to give us immunity against a different version of the virus,” Dr. Priya Sampathkumar, infectious disease specialist at the Mayo Clinic, tells TODAY.com.
About 95% of the population already has some degree of immunity from prior infection or vaccination, says Mulligan. However, this has waned over time, and the virus has mutated. The new COVID vaccine will act to “update” the body’s immune memory so it can respond faster and stronger to the virus, he explains.
“Receiving an updated COVID-19 vaccine can provide enhanced protection against the variants currently responsible for most infections and hospitalizations,” the CDC said in a release.
When will the new COVID boosters become available?
Following the CDC director’s approval of the new recommendations on Tuesday evening, the vaccines are expected to become available by the end of the week, the agency said.
The shots will likely start to arrive at pharmacies and doctor’s offices in the coming days, but experts encourage people to call and check beforehand first to make sure they are in stock.
It’s unclear whether — and how many — people will get the new vaccines. Uptake of the earlier bivalent booster rolled out last fall was low, the experts note, especially among children. Ultimately, only 17% of Americans who were eligible received the bivalent shot, according to CDC data.
Am I eligible to get an updated COVID vaccine?
According to the FDA, the following people are eligible to get the updated COVID vaccine in September 2023:
- Individuals 5 and older (previously vaccinated or unvaccinated) are eligible to receive a single dose of an updated mRNA COVID-19 vaccine at least 2 months after receiving the last dose of any COVID-19 vaccine.
- Children ages 6 months through 4 years who have previously been vaccinated against COVID-19 are eligible to receive one or two doses of an updated mRNA COVID-19 vaccine.
- Unvaccinated children ages 6 months through 4 years are eligible to receive two doses of the updated Moderna COVID-19 vaccine or three doses of the updated Pfizer-BioNTech vaccine.
The updated vaccines are safe and effective, the FDA said, and its benefit-risk assessments have demonstrated that the benefits for people ages 6 months and older outweigh the risks.
Who should get the updated COVID booster?
The CDC recommends everyone ages 6 months and older get the updated COVID-19 vaccine. The universal recommendation means that anyone who is eligible can get the vaccines once they are available, and that they will not be rolled out in a staggered fashion or restricted to certain populations at first.
While the risk of severe COVID-19, hospitalization and death is highest among people who are 65 or older and individuals who are immunocompromised, COVID-19 can cause hospitalizations and deaths among young and healthy people as well, Mulligan stresses.
“While the vaccine is pretty good at preventing infection in the first couple of months after you get it, it’s very good at preventing severe disease, hospitalization and deaths many months after you get it,” Dr. Kawsar Talaat, co-director of clinical research at the Johns Hopkins Institute for Vaccine Safety, tells TODAY.com.
So even if you do still get COVID-19 after vaccination, the experts note that it’s much more likely to be mild.
“We know the risk of long COVID increases with each infection of COVID, and there is increasing data … that suggests vaccination can reduce the risk of long COVID,” says Sampathkumar, adding that this is another reason for young, healthy people to get vaccinated.
Additionally, vaccination also helps produce herd immunity, which protects vulnerable individuals. “If I am a young healthy person who is vaccinated and either doesn’t get COVID or only has mild COVID, I’m less likely to transmit it to people around me,” says Mulligan.
When is the best time to get the new COVID booster?
For many people, the best time to get the new booster, according to the CDC, will be as soon as it becomes available. High-risk individuals should not hesitate to get the shot, the experts note.
However, if you’ve recently recovered from a COVID-19 infection, the experts recommend waiting a few months to get the updated vaccine. The CDC has previously said people with a recent infection should consider delaying vaccination by 3 months.
“You (want) the immune system to go back into its resting state. … In order for the updated vaccine to be most effective, you want to have mostly recovered from prior infection,” says Mulligan.
Also, if you recently got a previous COVID-19 vaccine or the bivalent booster, the FDA recommends waiting at least two months before getting the updated vaccine.
Individuals who are both high-risk and recently got the previous bivalent booster or recovered from infection should talk to their doctor about the best time to get the updated shot, the experts note.
It is safe to get the updated COVID vaccine and the seasonal flu shot at the same time, says Mulligan, who actually recommends knocking out both shots during the same trip to the doctor or pharmacy. “People are busy and it’s efficient, so having the option of a ‘twofer’ is a great thing,” he adds.
Side effects of new COVID vaccine
Side effects from the vaccine can vary from person to person, says Mulligan. According to the CDC, the most common side effects from COVID-19 vaccination include:
- Pain, redness or swelling an injection site on arm
- Fatigue
- Muscle aches
- Headaches
- Chills
- Low-grade fever
“Most of us have had several prior doses of the COVID vaccine, so it’s anticipated that the side effects will be similar to what you had with the prior doses or potentially less severe,” says Sampathkumar.
Side effects will typically go away within a day or two, Mulligan notes, and these can be managed with supportive care like rest, fluids and over-the-counter pain relievers. “If there’s concern about the side effects or feeling bad for a day or two, what I recommend is to get the vaccine on a Friday or the weekend,” says Mulligan.
Is the booster free or will it cost money?
Since 2020, the cost of COVID vaccines was covered by the federal government, and every person in the U.S. was able to get jabbed for free regardless of insurance coverage. The updated vaccine will not be provided free by the government, the experts note.
Fortunately, the majority of Americans will still be able to get the new shot for free, the CDC said in a release.
Most private and public insurance plans, including Medicare, will cover the cost of the updated vaccine, says Mulligan. However, people may need to visit an in-network provider in order to pay no out-of-pocket costs.
Pfizer and Moderna have said they are pricing each vaccine dose at over $100, NBC News previously reported.
If your health plan does not cover the cost of the vaccine, or you are one of the approximately 30 million Americans who does not have health insurance, there are still options. According to the CDC, uninsured individuals can get a free vaccine at local health clinics, state, tribal, or territorial health departments, and participating pharmacies.
How many COVID boosters will we need?
Barring the emergence of a significantly more contagious variant in the near future, the FDA said it anticipates that the COVID-19 vaccines will need to be updated annually.
Just like the flu vaccine is tweaked every year to better match the strains expected to circulate that season, the COVID-19 vaccine will likely be reformulated each year to better match the new, mutated strains circulating, the experts note.
“You get a new (flu shot) every year because the virus has changed so much in the intervening months that your prior vaccine no longer works,” which may also be the case with COVID in the future, Sampathkumar explains.
While it’s unclear how soon the vaccine will need to be reformulated, experts say we can anticipate COVID vaccines, and COVID, are here to stay.
Read the full article here